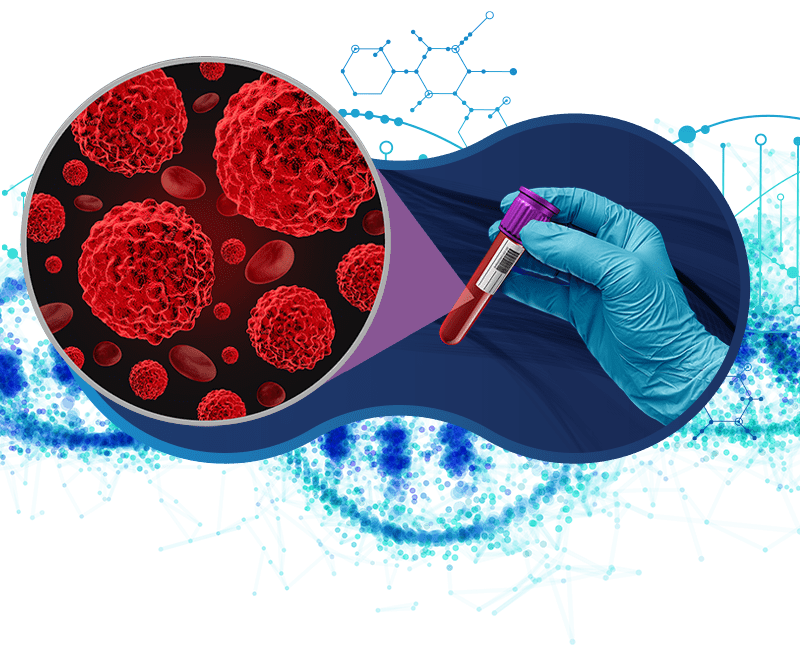
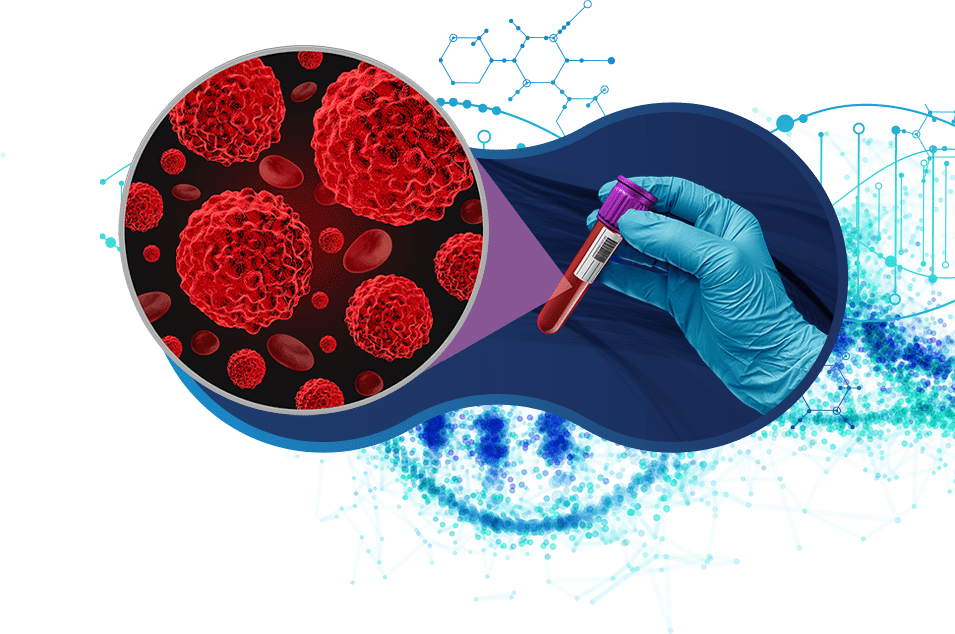
By detecting emerging mutations in ctDNA, liquid biopsy can identify signs of therapeutic resistance before they become clinically apparent. This proactive approach allows for timely adjustments in treatment, improving patient outcomes.
Discover the benefits of non-invasive liquid biopsy testing. Contact us for a consultation.

 Consult our Experts to find the right test for you.
Consult our Experts to find the right test for you.























Thank you for reaching out to us. Our team will get in touch with you shortly.

