Well-validated Comprehensive Genomic Profiling Kit (RUO) developed by experts to identify the genomic alterations (single nucleotide variations (SNVs), indels, copy number variants (CNVs)) and signatures across pan-solid cancers from wide sample sources.
Delivers/Uncovers molecular insights from cancer patient’s samples by focusing on therapeutic, prognostic, and diagnostic biomarkers from a single assay, which makes it efficient and rapid.
TARGT CGP Kit (RUO) includes reagents for hybridization-based library preparation (target enrichment and library creation) which can be used even for low sample input.
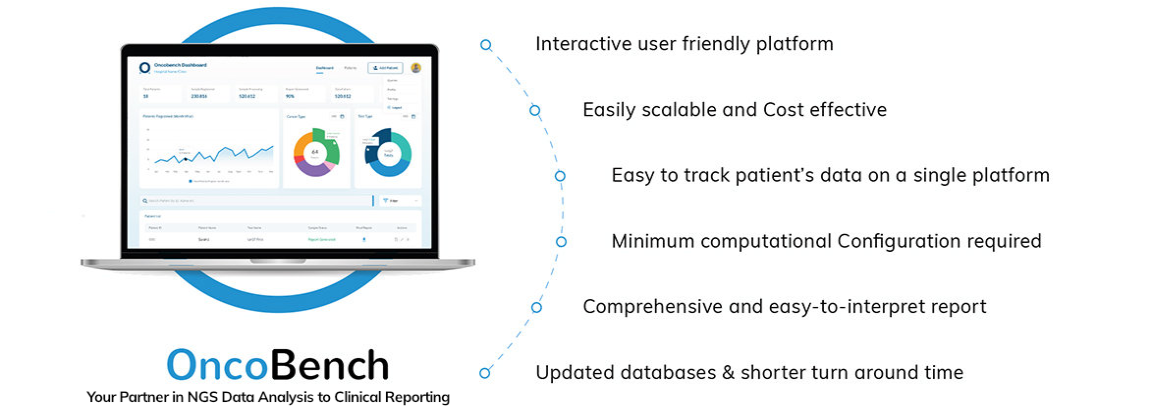
4baseCare Clinical Informatics platform: OncoBench supports your data analysis and reporting needs and enables quality control. The expert customer support team helps you to set up your tests and secure your results at every step.









 Kindly fill the form below to download our Liquid Biopsy Portfolio brochure :
Kindly fill the form below to download our Liquid Biopsy Portfolio brochure :